Hi SAT Aspirants, welcome to AKVTutorials. As you know SAT (Scholastic Assessment Test) is a standard test, used for taking admission to undergraduate programs of universities or colleges of United States. SAT is developed and published by the College Board, an organization in United States, administered by the Educational Testing Service. Therefore, you need to do practice on SAT Reading Section, SAT Writing and Language Section. In this article, you will get SAT Writing Section Questions Practice Test 69 with Answer Keys AMBIPi
Instruction:
- In the passage below is accompanied by a number of questions.
- For some questions, you need to think how the passage might be revised to improve the expression of ideas.
- For other questions, you will consider how the passage might be edited to correct errors in sentence structure, usage, or punctuation.
- Some questions will direct you to an underlined portion of a passage.
- Other questions will direct you to a location in a passage or ask you to think about the passage as a whole.
SAT Writing & Language Section Passage
SAT Writing Section Questions Practice Test Passage Title: The Effects of Electronegativity
1 What principles dictate the relationships between molecules? There are many factors at work, but these interactions also depend indirectly on a principle known as electronegativity. Although this is an atomic property, it generates molecular 2 forces that cause many of the phenomena we observe every day.
3 Electronegativity, the measure of an atom’s affinity for electrons, generally determines the type of bond present between two atoms. A single bond between atoms consists of two electrons. If the two atoms have similar electronegativities, they share the two electrons equally and form a nonpolar covalent bond. If two atoms have significantly differing values of electronegativity, there are two possible bond types: polar covalent and ionic. While other factors also 4 corrupt the determination of bond type, a difference in electronegativity between the values of 0.5 and 1.6 usually 5 result in a polar covalent bond, while a difference of more than 2.0 usually results in an ionic bond.
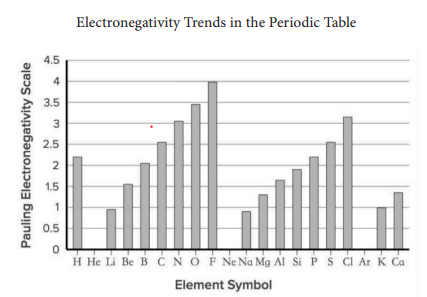
In a polar covalent bond, the two bonding electrons are 6 shared 6 unequally, in an ionic bond: both electrons are completely transferred to the more electronegative atom. For example, the bond between oxygen (O) and hydrogen (H) is classified as a polar covalent bond, because they share the two bonding electrons unequally. This polar bond type is partially caused by the difference in electronegativities: 7 hydrogen has an electronegativity of 2.20, while oxygen has an electronegativity of 3.44. When the electronegativity of the hydrogen is subtracted from the electronegativity of the oxygen, the difference is 1.24.
When a hydrogen atom is bonded to nitrogen, oxygen, or fluorine, this particular polar covalent bond makes that a new type of interaction possible: hydrogen bonding. For instance, a water molecule consists of an oxygen atom bonded to two hydrogen atoms. The unequal sharing of electrons causes a distribution of partial charges on the molecule. The oxygen of one water molecule monopolized the bonding electrons and acquires a partial negative 8 charge; as a result, this oxygen atom is attracted to the partially positive hydrogen atoms of another water molecule.
This electrostatic attraction, 9 which is an attraction referred to as intermolecular hydrogen bonding, contributes to the surface tension that sustains the weight of water striders and some other insects.
10 The presence of surface tension in water is just one result of the hydrogen bonding. From digestion to DNA structure, this molecular force is integral 11 with many life-sustaining processes. If atoms did not have different values of electronegativity, hydrogen bonding would be impossible—and life as we know it could not exist.
SAT Writing Section Questions Practice Test Questions
Question No 1
The writer wants to introduce the topic of electronegativity with a concrete, casually observable example from the natural world.
Which choice best accomplishes this goal?
Option A : No Change
Option B : How do water striders skim across the surfaces of ponds and lakes? Their hydrophobic legs are uniquely suited to this process, but the insects
Option C : How do our bodies break down the food we consume every day? While digestion would be impossible without enzymes and other proteins, these molecules
Option D : How does our DNA maintain a double helical structure? While the shape of this nucleic acid is the result of many complex properties, its structures
Answer
Show/Hide Answer
Option B: How do water striders skim across the surfaces of ponds and lakes? Their hydrophobic legs are uniquely suited to this process, but the insects
Question No 2
Which of the following options is the most effective?
Option A : No Change
Option B : forces—
Option C : forces
Option D : forces;
Answer
Show/Hide Answer
Option C : forces
Question No 3
The writer is considering deleting the underlined sentence. Should the writer make this deletion?
Option A : Yes, because the sentence unnecessarily repeats a definition provided earlier in the passage.
Option B : Yes, because the sentence is not relevant to the paragraph’s discussion of historic experiments that depended on electronegativity
Option C : No, because the sentence introduces the paragraph’s discussion of the relationship between electronegativity and bond type.
Option D : No, because the sentence provides an effective transition to the paragraph’s explanation of hydrogen bonding.
Answer
Show/Hide Answer
Option C : No, because the sentence introduces the paragraph’s discussion of the relationship between electronegativity and bond type.
Question No 4
Which choice results in the most effective transition to the information that follows in the paragraph?
Option A : No Change
Option B : impress
Option C : convince
Option D : influence
Answer
Show/Hide Answer
Option D : influence
Question No 5
Which choice best maintains the sentence pattern already established in the paragraph?
Option A : No Change
Option B : are resulting
Option C : results
Option D : have resulted
Answer
Show/Hide Answer
Option C : have resulted
Question No 6
Which choice best maintains the sentence pattern already established in the paragraph?
Option A : No Change
Option B : unequally; in the latter,
Option C : unequally, in the latter
Option D : unequally in the latter
Answer
Show/Hide Answer
Option B : unequally; in the latter,
Question No 7
Which choice most effectively uses accurate and relevant data from the graph in the passage to illustrate the concept being explained?
Option A : No Change
Option B : at 5.5, hydrogen has one of the highest electronegativity numbers on the Pauling electronegativity scale.
Option C : the electronegativity of oxygen is 3.44, a value significantly lower than that of hydrogen.
Option D : because their electronegativities lie in the 3 to 3.5 range, both oxygen and nitrogen are capable of helping cause a force known as ‘hydrogen bonding.
Answer
Show/Hide Answer
Option A : No Change
Question No 8
The writer is considering deleting the underlined sentence. Should the sentence be kept or deleted?
Option A : NO CHANGE
Option B : charge, this attracts
Option C : charge, as a result, this oxygen atom is attracted to
Option D : charging; attracting
Answer
Show/Hide Answer
Option A : NO CHANGE
Question No 9
Which choice best maintains the sentence pattern already established in the paragraph?
Option A : No Change
Option B : an attraction which is referred to as
Option C : a type of hydrogen bonding referred to as
Option D : referred to as
Answer
Show/Hide Answer
Option D : referred to as
Question No 10
Which choice provides the smoothest transition from the previous paragraph to this one?
Option A : No Change
Option B : Although the effects of hydrogen bonding are key, water striders also depend on the hydrophobic structure of their legs to keep them on top of the water’s surface.
Option C : Electronegativity is just one of the factors that determines the type of bond between two atoms.
Option D : Although hydrogen boning is a fascinating molecular force, the behavior of water also depends on the subatomic forces within each atom.
Answer
Show/Hide Answer
Option A : No Change
Question No 11
Which choice completes the sentence with accurate data based on the graph?
Option A : No Change
Option B : to
Option C : upon
Option D : into
Answer
Show/Hide Answer
Option B : to



